Revisiting cold-stored platelets: An effort to improve patient care and storage feasibility
Tuesday, March 29, 2022 Marie-Soleil Smith

This post was written by Marie-Soleil Smith, PhD Candidate in Dr. Hélène Côté’s Lab at the University of British Columbia, and edited by Dr. Geraldine Walsh, knowledge broker at Canadian Blood Services. It originally appeared on the Centre for Blood Research blog in March 2022.
Have you ever thought about how your blood is stored after you donate it? Since most of the readership of this article will likely be blood researchers, I’m sure the answer is yes. Platelets have been effectively stored the same way for decades, but is there a better way? There are numerous tasks that are a part of your day-to-day that you do a certain way because that is the way it has always been done. Have you ever considered questioning the status quo and finding out if the way we do it now, and the way we’ve been doing it for years, is really the best way?
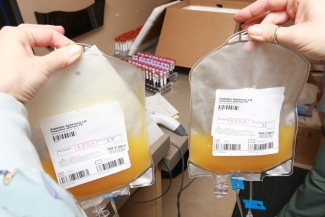
In this interview I caught up with Wayne Zhao, a PhD candidate in Dr. Dana Devine’s lab, to learn about his recent article on platelet storage conditions, both cold-stored and room-temperature, and how these conditions may impact the efficacy of platelets for certain uses.
-
Read the paper, published in the Journal of Proteome Research: In Vitro Characterization and Metabolomic Analysis of Cold-Stored Platelets
Tell me about your study. What was it about and what were the main findings?

Cold-stored platelets were used back in the 1960s but their use was stopped because they were less effective for people who needed prophylactic transfusions; for example, those who have chronically low platelets due to chemotherapy. The problem with cold-stored platelets is that they don’t stick around in the patient’s body after you transfuse them. So, patients who needed prophylactic platelet transfusions would have to come back every single day, whereas with room-temperature platelets, these patients only require transfusions every three to four days.
Our study took another look at cold-stored platelets, as there have been interesting new reports in the literature indicating that cold-stored platelets are an effective short-term fix when it comes to people who are actively bleeding, like someone who has just experienced a traumatic injury or is undergoing childbirth complications. The main finding of our study is that the in vitro storage characteristics of cold-stored platelets are very similar to what other investigators have found. They have better physiological responses, they aggregate better, and they can be stored for up to 14 days compared to the shorter 7-day shelf-life of room-temperature platelets.
In addition, we did a metabolomics study to help understand what temperature does to platelet metabolites. When platelets clot in the body, they release all kinds of different metabolites, like lactate, or the antioxidant glutathione. We looked at approximately 200 metabolites and found that many are significantly affected by temperature. In the case of glutathione, we noticed that there is a consistent level of it in cold-stored platelets, whereas the room-temperature platelets consume glutathione very quickly. This suggests to us that room-temperature platelets experience more oxidative stress, which may be why their storage quality isn’t as good as cold storage. There is much more work to be done with these metabolites, as many have distinct differences between cold and room-temperature stored platelets – we’ve just scratched the surface.
What methods did you use to conduct the study? Why did you use these methods?
The platelet pools were generated by the buffy coat method by Canadian Blood Services, so the platelets that we used are the same as those used for transfusions in hospitals in Canada. Using the buffy coat method to generate platelets is different from what is done in the US, where they use apheresis platelets. Buffy coat platelets are generated from whole blood donations. To make one unit of platelet concentrate, you need to pool buffy coats from four donors together, whereas an apheresis platelet unit is from just one donor.
Many of the assays we used are standard and used throughout the world. We used hematology analyzers to measure platelet count, platelet volume, and other parameters such as blood gas, lactate content, glucose concentration, pCO2, pO2, calcium concentration, and etcetera. We also used flow cytometry to look at markers of platelet activation.
The metabolomics methods are a bit more complicated, so we collaborated with Angelo D'Alessandro’s group in Denver. They ran high-pressure liquid chromatography (HPLC) in tandem with their metabolomic machine. I’m not an expert on metabolomics, so it was very useful to have collaborators. The data analysis of the metabolic study was the most difficult part. You generate a lot of data with these studies so it’s difficult to sort out real differences versus the background.
What is the biggest contribution of this study to the existing literature?
The biggest contribution would be the final figure of the paper where we link the in vitro study with our metabolomics study. Many groups have done the in vitro portion, and others have done the metabolomics, but what we really wanted to find out was how these metabolites affect in vitro changes in storage quality. To do that, we looked at the correlation between the metabolite changes and our in vitro work and we noticed that there were a couple of important pathways that impact storage quality.
One of the pathways that I mentioned before was the reactive oxygen pathway. This pathway is very important because it seems that when the platelets are being stored, they experience a lot of oxidative stress, especially platelets stored at room temperature. At room temperature the platelets are active, so they make a lot of free radicals, and thus they experience a lot of oxidative stress. Another key pathway is the pentose phosphate pathway. We saw that this pathway is highly activated in room temperature, but not cold-stored platelets. This gave us a good indication of which pathways are important when it comes to storage. We also looked at the platelets and how they clot using rotational thromboelastometry (ROTEM). We noticed that ADP and ATP are important for clot strength. This is somewhat expected because platelets need energy from ATP to do their work, so that’s another important pathway that we looked at.
What are the limitations of this study? How might the limitations be addressed in a future project?
One of the limitations of our study was suggested by one of the reviewers of the manuscript. The buffy coat platelets that we analyzed are stored in plasma. This brings up a limitation as we are unable to tell whether the metabolites we looked at came from platelets or from the plasma. From a purely scientific point of view, identifying plasma- versus platelet-derived metabolites is an important distinction that we didn’t address, but fortunately the goal of our study was to address what happens in platelet units that are used for transfusion in Canada, which are stored in plasma.
Another limitation is that we do not know whether the metabolites came from inside the platelets, or if they were released from the platelets into solution. To add to that, we don’t know if the metabolites came from the organelles or the cytoplasm. These distinctions are very important, take the tricarboxylic acid (TCA) cycle for example, if we see a lot of TCA metabolites in the cytosol then that indicates that the mitochondria are failing. We don’t know the orientation or the origin of the metabolites we looked at, so it’s hard to make conclusions about such things as mitochondrial failure.
What are the future directions for this project?
Next, we will be focusing on the different pathways I mentioned previously. The ultimate goal of this project is to see if we can improve platelet storage in some way and I think this metabolomics study opened up a lot of different directions. We are now looking into inhibiting the pentose phosphate pathway to see if this prevents activation in both cold and room-temperature platelets, which will further support our hypothesis that this pathway is important in storage. Beyond that, we will take advantage of the pathway to see if we can improve platelet storage quality.
If you were in a decision-making position within a blood organization, how would you store platelets?
The first thing I would do is run a clinical trial. Let’s assume the clinical trial is successful and Health Canada agrees that cold-stored platelets are useful. I would then set up a dual inventory system of platelets for transfusion. We won’t be transitioning back fully to cold-stored platelets, as 70% of people needing a transfusion are those with a chronic need for platelets who will continue to need room-temperature platelets. The dual system would be useful since cold-stored platelets can be used for people who come in with traumatic injuries and active bleeding episodes since these are the patient who may benefit from cold-stored platelets. The other benefit is that the cold-stored product would be able to be stored for twice as long as room temperature-stored platelets. This would drastically increase platelet shelf-life for this part of the inventory. Additionally, remote hospitals in the interior or the northern parts of the provinces could have cold-stored platelets on the shelf, which could help with the logistical challenges and costs associated with ensuring these hospitals can provide platelets to patients.
What is the take-home message of your work?
We are revitalizing an old product to improve patient care.
How did you feel after the experiments were done and the manuscript was complete?
After the experiments were done, I felt relieved, but to be honest after the manuscript was complete, I was really worried. At the time we submitted our manuscript, the COVID-19 pandemic was just beginning. A lot of the investigators were busy reviewing COVID-related papers, so our paper was sort of set aside. I didn’t feel fully relieved until the editor sent us the acceptance letter.
Canadian Blood Services – Driving world-class innovation
Through discovery, development and applied research, Canadian Blood Services drives world-class innovation in blood transfusion, cellular therapy and transplantation—bringing clarity and insight to an increasingly complex healthcare future. Our dedicated research team and extended network of partners engage in exploratory and applied research to create new knowledge, inform and enhance best practices, contribute to the development of new services and technologies, and build capacity through training and collaboration. Find out more about our research impact.
The opinions reflected in this post are those of the author and do not necessarily reflect the opinions of Canadian Blood Services nor do they reflect the views of Health Canada or any other funding agency.
Related blog posts
Blood is red. That’s because of the red blood cells or erythrocytes that whizz around your veins and arteries. The colour is a great visual marker, both clinically and emotionally, but sometimes its very redness hides the other important components that are in you to give. These include plasma, the...
The Centre for Blood Research’s symposium was held in Vancouver, British Columbia in April. Featuring talks from world-class researchers, trainees and patients, the Norman Bethune Symposium provided attendees with the perfect blend of information and inspiration.