Permission to collect umbilical cord blood
What are cord blood stem cells?
Umbilical cord blood contains blood stem cells, which are immature cells that develop into these cells in the bloodstream:
- Red blood cells to carry oxygen in your body
- Platelets to help blood to clot
- White blood cells to fight infections
What can cord blood stem cells be used for?
The Cord Blood Bank collects, tests and stores cord blood stem cells to make them available for any patient in need of a stem cell transplant in Canada or worldwide. Occasionally, the Cord Blood Bank may use cord blood stem cells for other uses such as quality assurance (e.g. validation, training and quality control) and product improvement (“Other Uses”), and research through the Cord Blood Bank research program if you have checked off the "Research Opt-In" option (“Research Option").
How are cord blood stem cells collected?
Your healthcare team (e.g. physician, nurse, midwife) helps the Cord Blood Bank with the collection of cord blood. After your baby is born, the umbilical cord is clamped then cut, and the placenta, or “afterbirth,” is delivered and then transferred to the Cord Blood Bank staff at the hospital. The cord blood is then collected from the umbilical cord and checked for eligibility for storage with the Cord Blood Bank. Requests to have the placenta and/or umbilical cord returned to you are to be directed to your healthcare team.
Determining if the cord blood is eligible for the Cord Blood Bank
If it is determined at the hospital that the cord blood contains enough cord blood stem cells, it will be transferred to the Cord Blood Bank manufacturing facility for further assessment. Before you leave the hospital, a Cord Blood Bank staff member will speak with you about consent to store the cord blood for future transplant use. This includes asking questions about your medical history and general health. You will be asked to provide a blood sample which will be tested for blood groups (ABO and Rh), compatibility testing (to determine if the stem cells can be transplanted safely into a particular patient), HIV, hepatitis B and C, human T-cell lymphotropic virus (HTLV), Cytomegalovirus (CMV), West Nile virus, and if applicable, Chagas disease and other factors. We will report any positive test results for these diseases to you and your health care provider per Canadian Blood Services’ policy. If you require IV (intravenous) fluid during labour, we may ask that you provide the blood sample at that time. Staff will draw six small tubes (about three tablespoons) of blood from you.
If the cord blood is not eligible, depending on where you deliver, it may be
- discarded,
- transferred to the Cord Blood Bank manufacturing facility to be used for Other Uses or used under the Cord Blood Bank’s research program if you have checked off the Research Option, or
- returned to your hospital, to be dealt with according to approved hospital policies (including hospital research). Questions about these policies should be directed to a hospital representative.
Storage and Transplantation
Once the cord blood arrives at our manufacturing facility, we will start testing and preparing the cord blood stem cells for storage. The results of this testing will help us determine if the cord blood stem cells can be stored for future transplant use. If at any time, we determine that the cord blood stem cells may not be stored, the cord blood and any blood samples may be discarded, used for Other Uses, or used for biomedical research under the Cord Blood Bank’s research program if you have checked off the Research Option.
Research Option
If at any time, the Cord Blood Bank determines that the cord blood stem cells may not be stored for future transplant use, they could still be used for biomedical research under the Cord Blood Bank’s research program. In addition, by-products (such as red blood cells) that are removed from the cord blood during preparation for storage can be used for research rather than being discarded. Mark the Research Opt-In box so that the Cord Blood Bank may use or distribute the cord blood and by-products for biomedical research under the Cord Blood Bank’s research program. Please read the Canadian Blood Services’ Cord Blood Bank Information For Cord Blood Donation For Biomedical Research page.
You may be asked to participate in research by a hospital researcher who is not associated with the Cord Blood Bank, whether or not you checked off the Research Option.
Costs and Reimbursements
There is no cost to donate cord blood to the Cord Blood Bank. There is no reimbursement for any part of your cord blood donation.
Potential Benefits
Your donation may give a patient with a life-threatening disease a chance for a healthy life.
Withdrawing from the Cord Blood Bank
Your participation in the Cord Blood Bank is voluntary. You may withdraw at any time by calling 1 888 2 DONATE (1-888-236-6283). Your baby’s cord blood stem cells will be discarded if they have not already been used for transplantation, Other Uses or the Research Option, where applicable. The personal information Canadian Blood Services collected up to the date of your request for withdrawal will remain within Canadian Blood Services records but no further information about you or your baby will be collected, used or disclosed. Withdrawal of your consent will not affect the use and disclosure of personal information already collected by Canadian Blood services as outlined in our Privacy Notice.
*This form is to be completed by the birthing parent*
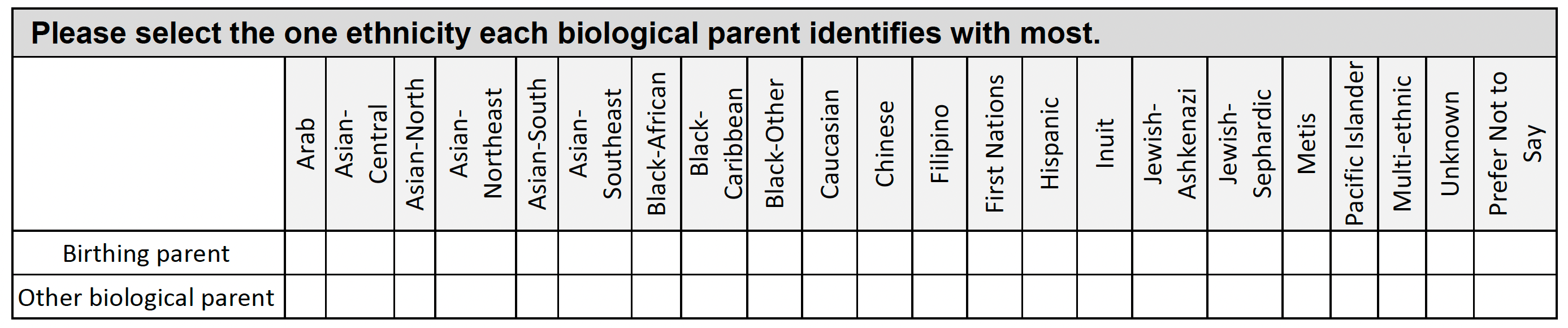
Ethnic Background
This information helps match a potential donor and a patient in need of a stem cell transplant.
Permission to Collect
I have read and understand the information about cord blood donation. I have had the opportunity to ask questions and I am satisfied with the answers to the questions. I have had access to an independent health care provider (e.g. my physician or midwife) for any questions or concerns regarding cord blood donation.
I voluntarily consent to the collection of cord blood after delivery for the purposes described in this form, to have a blood sample taken from me and to allow staff to review my medical record and my baby’s medical record. I have read, understand and consent to the collection, use and disclosure of my personal information and my baby’s personal information as outlined in the Privacy Notice. I understand how I may withdraw my consent.
I understand that I may be asked to participate in research by a hospital researcher who is not associated with the Cord Blood Bank, whether or not I mark the Research Opt-In box.
|
Research Option: By marking the Research Opt-In box, I voluntarily agree to allow Canadian Blood Services to use or distribute my cord blood donation, if it is not eligible for storage, and by-products to researchers for the purposes of biomedical research under the Cord Blood Bank’s research program. I have read Canadian Blood Services’ Cord Blood Bank Information For Cord Blood Donation For Biomedical Research and have had the opportunity to ask questions. I understand that Canadian Blood Services will not notify me if the cord blood unit is used for biomedical research, and I will not receive results of any research using donated cord blood under the Cord Blood Bank’s research program. In the rare case that a research project reveals an unexpected finding that may be important to me, I understand that I may be contacted by Canadian Blood Services. I understand that research results will be posted on the Canadian Blood Services website. □ RESEARCH OPT-IN (mark the box if you agree to the Research Option) |
(Printed name)
(Signature)
(Date)
(Postal Code)
(Telephone Number)
(E-mail, optional)
| For CBB Use Only: |
|---|
| Change of Information: □ Name □ Address □ Postal Code □ Phone Number |
|
Verification of information discrepancy from unique hospital unique ID label to documented information by birthing parent: |
|
CBU Unique ID Number
|