Diagnostic Services Alberta Year In Review 2021
Senior Staff and Contact Information
| Laboratory Medical Director 780-431-8714 Dr. Judith Hannon, MD, FRCPC judy.hannon@blood.ca |
Diagnostic Services Manager 604-707-3449 Lhevinne Ciurcovich, MLT lhevinne.ciurcovich@blood.ca |
|
Technical Supervisor Diagnostic Services |
Supervisor, Testing |
|
Supervisor, Genotyping |
Supervisor, Operational Support |
|
Supervisor, Operational Support |
Perinatal Laboratory |
|
Reference Laboratory |
Laboratory Services Website https://blood.ca/en/hospital-services/laboratory-services |
Figures
- Figure 1: Total Number of Perinatal Specimens Tested between 2017 and 2021
- Figure 2: Total Number of Clinically Significant Perinatal Antibodies Detected between 2017 and 2021
- Figure 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
- Figure 4: Total Reference Specimens Tested by Edmonton Reference Laboratory between 2017 and 2021
- Figure 5: Total Number of Reference Antibodies Detected between 2017 and 2021
- Figure 6: Frequency of Reference Antibodies Detected between 2017 and 2021
- Figure 7: RhD Testing Algorithm
- Figure 8: Turnaround Time for Perinatal Routine Samples between 2017 and 2021
- Figure 9: Turnaround Time for Reference Testing between 2017 and 2021
- Figure 10: Perinatal Rejection Reasons in Year 2021
- Figure 11: Reference Rejection Reasons in Year 2021
Tables
- Table 1: Total Number of Perinatal Specimens and Patients Tested between 2017 and 2021
- Table 2: Total Number of Clinically Significant and Insignificant Perinatal Antibodies Detected between 2017 and 2021
- Table 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
- Table 4: Perinatal Patient Antibody Titres Performed in Year 2021 and changed from Non-critical Level to Critical Level
- Table 5: Combination Antibodies Detected in Perinatal Patients in 2021
- Table 6: Total Reference Specimens Tested by Edmonton Reference Laboratory between 2017 and 2021
- Table 7: Total Number of Reference Antibodies Detected between 2017 and 2021
- Table 8: Frequency of Reference Antibodies Detected between 2017 and 2021
- Table 9: Combination Reference Antibodies Detected in 2021
- Table 10: Fetal Genotyping Results Summary between 2017 and 2021
- Table 11: Fetal Genotyping Results Summary from year 2021
- Table 12: Patient # - RHD Type/Result in year 2021
- Table 13: RHD Genotyping – Number of Rh Positive and Rh Negative Predicted Phenotypes Between 2017 and 2021
- Table 14: Turnaround Time from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available – Routine Criteria by Specimen Type
- Table 15: Turnaround Time from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available – Perinatal Routine TAT between 2017 and 2021
- Table 16: Turnaround Time from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available – Reference Specimens TAT between 2017 and 2021
- Table 17: Turnaround Time for Perinatal Routine Samples between 2017 and 2021
- Table 18: Turnaround Time for Reference Testing between 2017 and 2021
- Table 19: Quarterly Rejection Rates – Perinatal Specimens in Year 2021
- Table 20: Quarterly Rejection Rates – Reference in Year 2021
Perinatal Laboratory
The Perinatal Laboratory within Diagnostic Services at Canadian Blood Services provides diagnostic testing of perinatal samples for blood type and red blood cell antibodies. Results from this screening assist physicians, midwives, and nurse practitioners in ensuring the appropriate management of a pregnancy for both the patient and baby.
A. Testing Performed
Canadian Blood Services Perinatal Laboratory routinely performs the following tests:
- ABO/Rh blood type
- Screen for red blood cell antibodies
- Antibody Identification
- Antibody Titration
- Phenotyping
- Fetal Bleed Screening Test
- Kleihauer-Betke Test for quantitation of fetal-maternal hemorrhage
- Postnatal Testing
Automated ABO/Rh, Antibody Screen, and Antibody Identification assays are routinely performed on the Immucor NEO Iris analyzer (hemagglutination testing and solid phase). Manual follow up testing includes the use of PEG, LISS and other methods.
B. Testing Frequency
Prenatal – Initial Testing: All patients should be tested upon their first prenatal visit.
Prenatal – 26-28 Weeks Gestation: All Rh-negative patients should be retested at 26-28 weeks gestation. Rh positive patients should also be retested at 26-28 weeks gestation when there is only one blood group result available (usually first pregnancy) or if patient is at increased risk of allo-immunization (e.g., previous transfusion, trauma, or obstetrical procedure).
Prenatal – Antibody Present: If the antibody is known to cause HDFN, it is recommended that specimens be submitted monthly in the first and second trimester and every two weeks in the last trimester. More frequent testing may be indicated if the antibody titre rises rapidly or if clinical monitoring mandates that additional sampling would provide helpful information. Less frequent sampling may also be recommended for antibodies that are unlikely to be clinically significant or in cases where clinical monitoring through fetal Doppler ultrasound has commenced.
Postnatal: Following delivery, specimens from the patient and baby should be tested if the Rh of the patient is unknown, the patient is Rh negative, the patient has a clinically significant antibody or if the baby shows signs of HDFN (i.e., anemia or jaundice). Midwives or hospitals that do not perform transfusion medicine testing should submit specimens to Canadian Blood Services. A fetal bleed screening test is performed if an Rh-negative patient delivers an Rh positive baby. The Kleihauer-Betke assay is performed when the patient has a positive fetal bleed screening test.
Newborns (Cords): Cord blood or neonatal specimens must be submitted with the patient’s specimen as noted above. ABO/Rh and direct antiglobulin testing is performed on cord or neonatal specimens submitted to Canadian Blood Services. The direct antiglobulin test is performed if the patient has a clinically significant antibody or on request if the baby shows signs of HDFN (i.e., anemia or jaundice). This is especially important when the patient is Rh negative or when the patient has a clinically significant antibody. If the baby has unexpected anemia or jaundice assessment of the cord blood sample for blood group and DAT may also be helpful.
Partners: When a prenatal patient has an antibody capable of causing HDFN, specimens from the partner will be requested for ABO/Rh and antigen phenotyping. This will assist in assessing the probability of the baby being affected by the antibody. Partners’ specimens may also be tested to assess Rh Immune Globulin (RhIG) eligibility of Rh-negative patients.
C. Specimens Tested
The data includes all perinatal patients tested. The data in this report reflects a calendar year period to enable better correlation to other government statistical data (Statistics Canada birth statistics).
Figure 1: Total Number of Perinatal Specimens Tested between 2017 and 2021
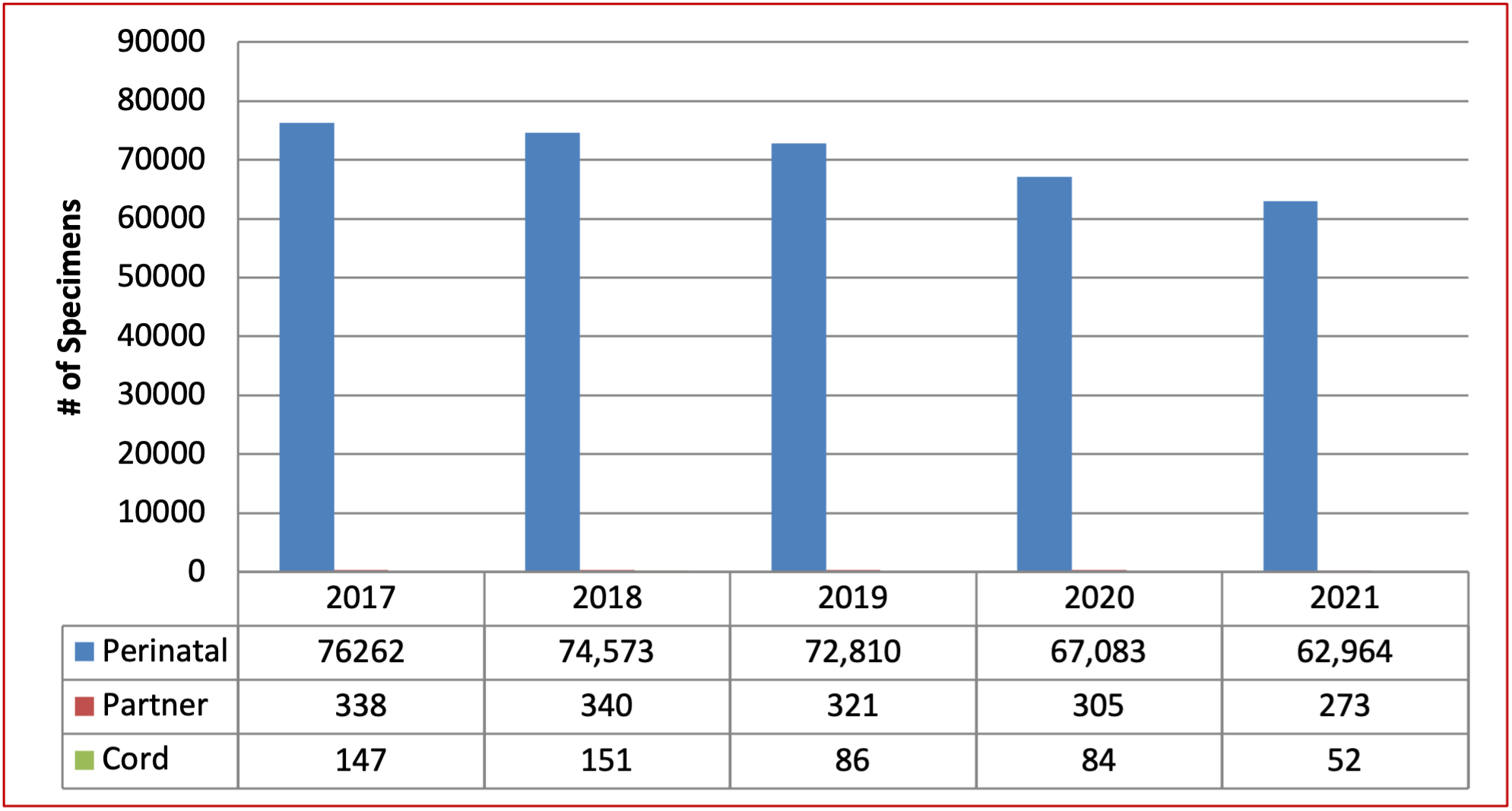
Table 1: Total Number of Perinatal Specimens and Patients Tested between 2017 and 2021
|
Specimen Type |
Test Type |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|---|
|
Perinatal |
Type and Screen |
76,262 |
74,573 |
72,810 |
67,083 |
62,964 |
|
Partner |
ABO/Rh |
338 |
340 |
321 |
305 |
273 |
|
Cord |
ABO/Rh |
147 |
151 |
86 |
84 |
52 |
|
Total # of Specimens Tested |
|
76,747 |
75,064 |
73,217 |
63, 289 |
|
|
Total # of Patients Tested |
Not reported |
Not reported |
Not reported |
60,639 |
55, 091 |
|
Text Version - Table 1
Total number of perinatal specimens and patients tested between 2017 and 2021 includes specimen types Perinatal, Partner and Cord. Perinatal specimens were tested for Type and Screen. Total number of perinatal specimens tested in 2017 were 76262, in 2018 – number of perinatal specimens were 74573, in 2019 – number of perinatal specimens were 72810, in 2020 – number of perinatal specimens were 67083 and in 2021, number of perinatal specimens were 62964. Partner specimens were tested for ABO/Rh. Total number of partner specimens tested in 2017 were 338, in 2018 – number of partner specimens were 340, in 2019 – number of partner specimens were 321, in 2020 – number of partner specimens were 305 and in 2021, number of perinatal specimens were 273. Cord specimens were also tested for ABO/Rh. Total number of cord specimens tested in 2017 were 147, in 2018 – number of cord specimens were 151, in 2019 – number of cord specimens were 86, in 2020 – number of cord specimens were 84 and in 2021, number of cord specimens were 52. Total number of patients tested were not reported in years 2017, 2018 and 2019. Total of 60639 patients were tested in 2020 and 55091 patients were tested in 2021.
D. Antibodies Identified
In 2021, a total of 424 antibodies were reported (see Table 2). This is higher than 2020 where 353 antibodies were reported. Of 424 antibodies identified in 2021, seventy-eight (78) patients had multiple antibodies. Passive anti-D data has been excluded from the preceding numbers.
Antibodies identified are considered to be clinically significant if they have been reported to cause HDFN. The most common clinically significant antibodies identified were: anti-E, anti-c, anti-D, anti-K, anti-M (IgG), (see Figure 2) which together represented 66% of the total antibodies identified. IgG Anti-M can be considered clinically significant as it may cause HDFN and/or delayed neonatal anemia in rare cases.
Titres for 13 of the clinically significant antibodies increased from non-critical to critical levels during the pregnancy with a total of 47 antibody titres at critical levels (see Table 4). Recommendations were made for all patients with a critical titre level (current or previous pregnancy) and all Kell system antibodies to be referred to a High-Risk Fetal Assessment Clinic for further follow-up and monitoring during pregnancy.
Figure 2: Total Number of Clinically Significant Perinatal Antibodies Detected between 2017 and 2021
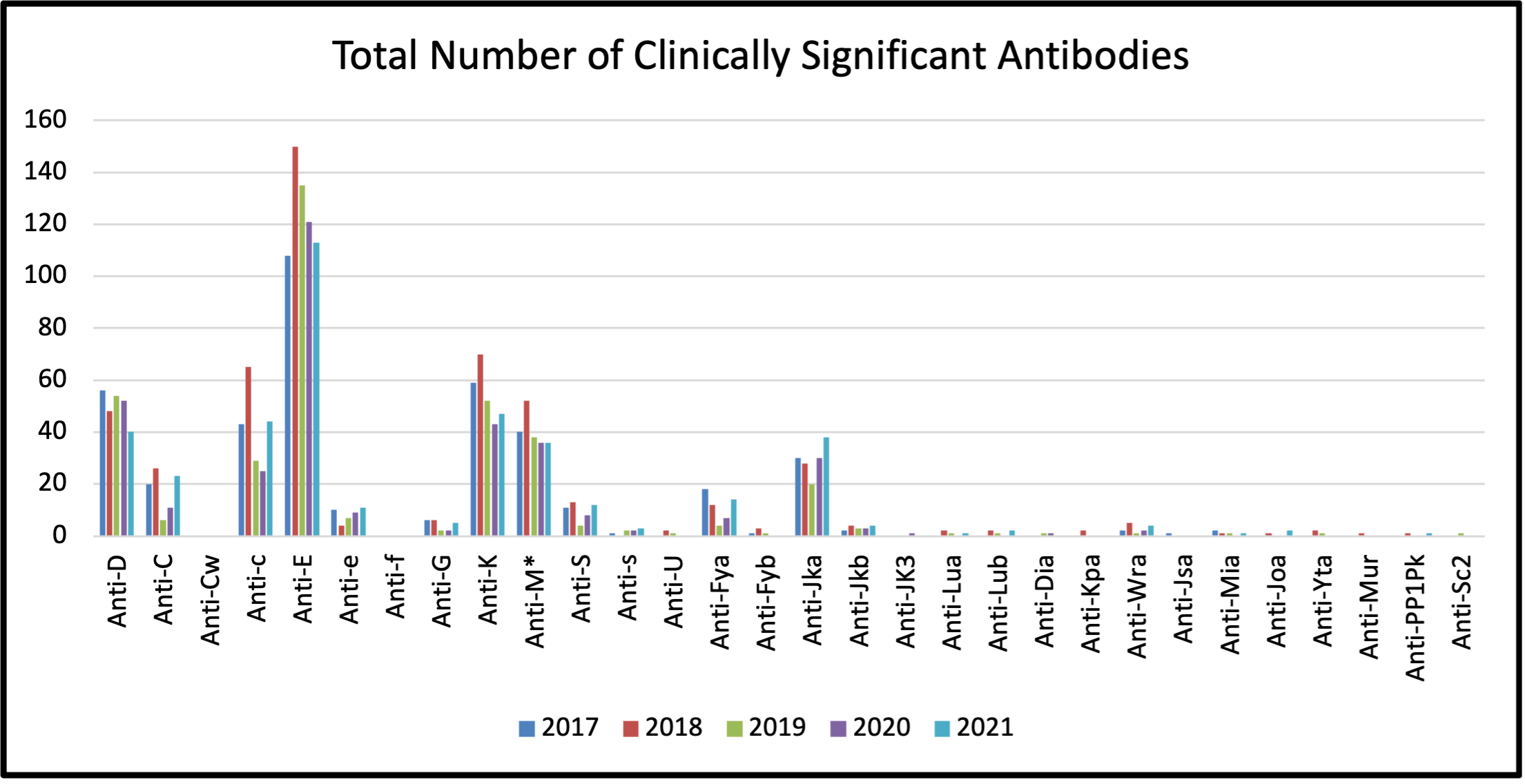
Table 2: Total Number of Clinically Significant and Insignificant Perinatal Antibodies Detected between 2017 and 2021
|
Perinatal Antibodies Identified |
|||||
|---|---|---|---|---|---|
|
Clinically Significant Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|
Anti-D |
56 |
48 |
54 |
52 |
40 |
|
Anti-C |
20 |
26 |
6 |
11 |
23 |
|
Anti-Cw |
0 |
0 |
0 |
0 |
0 |
|
Anti-c |
43 |
65 |
29 |
25 |
44 |
|
Anti-E |
108 |
150 |
135 |
121 |
113 |
|
Anti-e |
10 |
4 |
7 |
9 |
11 |
|
Anti-f |
0 |
0 |
0 |
0 |
0 |
|
Anti-G |
6 |
6 |
2 |
2 |
5 |
|
Anti-K |
59 |
70 |
52 |
43 |
47 |
|
Anti-M* |
40 |
52 |
38 |
36 |
36 |
|
Anti-S |
11 |
13 |
4 |
8 |
12 |
|
Anti-s |
1 |
0 |
2 |
2 |
3 |
|
Anti-U |
0 |
2 |
1 |
0 |
0 |
|
Anti-Fya |
18 |
12 |
4 |
7 |
14 |
|
Anti-Fyb |
1 |
3 |
1 |
0 |
0 |
|
Anti-Jka |
30 |
28 |
20 |
30 |
38 |
|
Anti-Jkb |
2 |
4 |
3 |
3 |
4 |
|
Anti-JK3 |
0 |
0 |
0 |
1 |
|
|
Anti-Lua |
0 |
2 |
1 |
0 |
1 |
|
Anti-Lub |
0 |
2 |
1 |
0 |
2 |
|
Anti-Dia |
0 |
0 |
1 |
1 |
|
|
Anti-Kpa |
0 |
2 |
0 |
0 |
0 |
|
Anti-Wra |
2 |
5 |
1 |
2 |
4 |
|
Anti-Jsa |
1 |
0 |
0 |
0 |
0 |
|
Anti-Mia |
2 |
1 |
1 |
0 |
1 |
|
Anti-Joa |
0 |
1 |
0 |
0 |
2 |
|
Anti-Yta |
0 |
2 |
1 |
0 |
0 |
|
Anti-Mur |
0 |
1 |
0 |
0 |
0 |
|
Anti-PP1Pk |
0 |
1 |
0 |
0 |
1 |
|
Anti-Sc2 |
0 |
0 |
1 |
0 |
0 |
|
Anti-Cob |
0 |
0 |
0 |
1 |
0 |
|
Anti-Dia |
0 |
0 |
0 |
0 |
3 |
|
Anti-Vel |
0 |
0 |
0 |
0 |
1 |
|
Anti-Hr |
0 |
0 |
0 |
0 |
1 |
|
Panreactive Autoantibody |
0 |
0 |
0 |
16 |
17 |
|
Antibody to a Low Prevalence Antigen |
0 |
0 |
0 |
1 |
1 |
|
Total |
410 |
500 |
364 |
353 |
424 |
*Anti-M – IgG antibody detected
|
Clinically Insignificant Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Anti-A1 |
|
|
10 |
2 |
8 |
|
Anti-Lea |
12 |
20 |
11 |
15 |
16 |
|
Anti-Leb |
1 |
3 |
3 |
2 |
1 |
|
Anti-N |
2 |
1 |
2 |
1 |
1 |
|
Anti-P1 |
1 |
2 |
1 |
0 |
1 |
|
Anti-VS |
0 |
0 |
0 |
0 |
0 |
|
Anti-Ytb |
0 |
0 |
0 |
0 |
1 |
|
Cold agglutinin |
0 |
0 |
0 |
9 |
8 |
|
0 |
0 |
0 |
21 |
23 |
|
|
Passive Anti-D (not included in totals) |
680 |
555 |
855 |
726 |
601 |
|
TOTAL: Clinically Insignificant Antibodies |
16 |
26 |
17 |
18 |
19 |
Figure 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
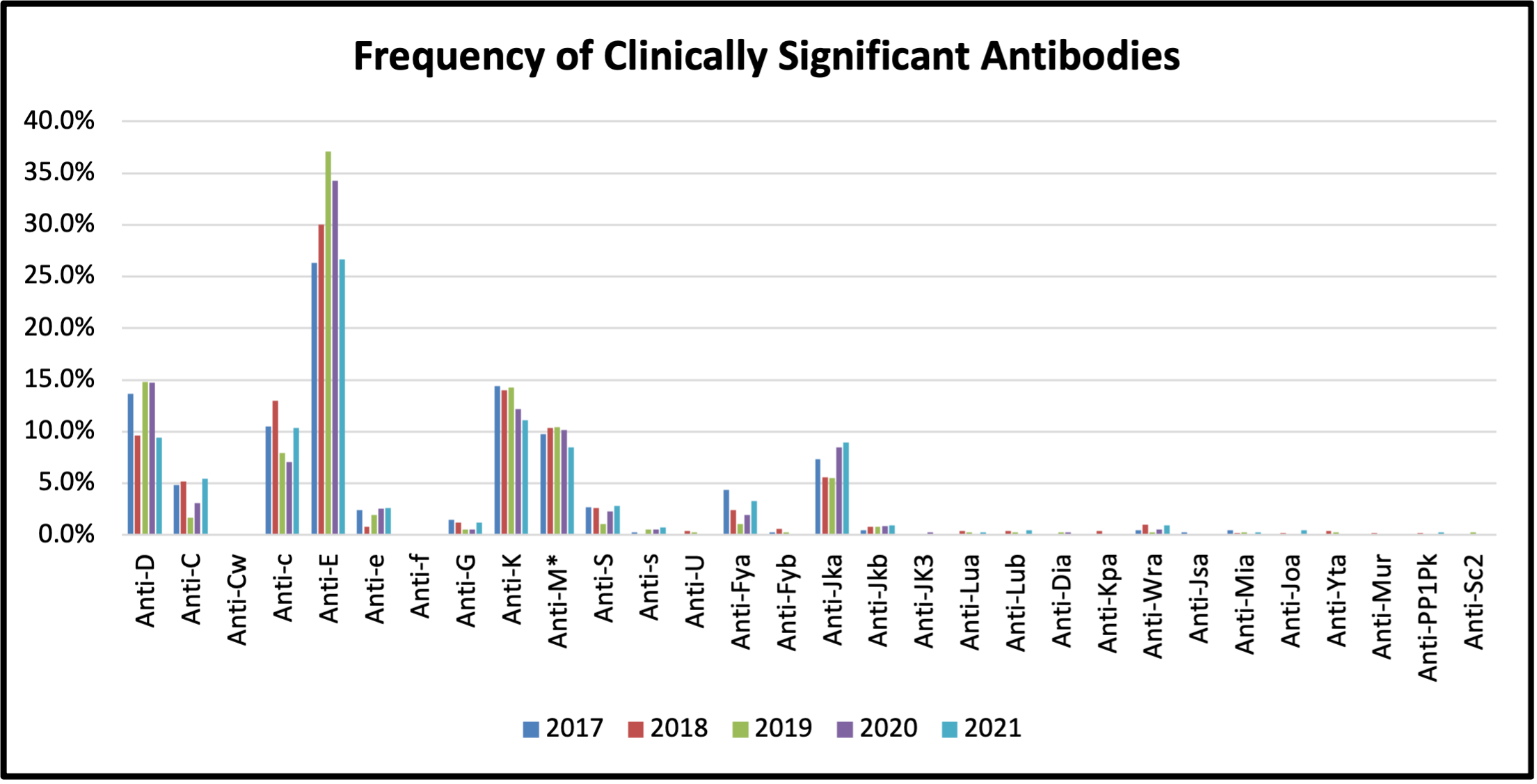
Table 3: Frequency of Clinically Significant Antibodies Detected between 2017 and 2021
|
Clinically Significant Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Anti-D |
13.7% |
9.6% |
14.8% |
14.7% |
9.4% |
|
Anti-C |
4.9% |
5.2% |
1.6% |
3.1% |
5.4% |
|
Anti-Cw |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-c |
10.5% |
13.0% |
8.0% |
7.1% |
10.4% |
|
Anti-E |
26.3% |
30.0% |
37.1% |
34.3% |
26.7% |
|
Anti-e |
2.4% |
0.8% |
1.9% |
2.5% |
2.6% |
|
Anti-f |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-G |
1.5% |
1.2% |
0.5% |
0.6% |
1.2% |
|
Anti-K |
14.4% |
14.0% |
14.3% |
12.2% |
11.1% |
|
Anti-M* |
9.8% |
10.4% |
10.4% |
10.2% |
8.5% |
|
Anti-S |
2.7% |
2.6% |
1.1% |
2.3% |
2.8% |
|
Anti-s |
0.2% |
0.0% |
0.5% |
0.6% |
0.7% |
|
Anti-U |
0.0% |
0.4% |
0.3% |
0.0% |
0.0% |
|
Anti-Fya |
4.4% |
2.4% |
1.1% |
2.0% |
3.3% |
|
Anti-Fyb |
0.2% |
0.6% |
0.3% |
0.0% |
0.0% |
|
Anti-Jka |
7.3% |
5.6% |
5.5% |
8.5% |
9.0% |
|
Anti-Jkb |
0.5% |
0.8% |
0.8% |
0.8% |
0.9% |
|
Anti-JK3 |
0.0% |
0.0% |
0.0% |
0.3% |
0.0% |
|
Anti-Lua |
0.0% |
0.4% |
0.3% |
0.0% |
0.2% |
|
Anti-Lub |
0.0% |
0.4% |
0.3% |
0.0% |
0.5% |
|
Anti-Dia |
0.0% |
0.0% |
0.3% |
0.3% |
0.0% |
|
Anti-Kpa |
0.0% |
0.4% |
0.0% |
0.0% |
0.0% |
|
Anti-Wra |
0.5% |
1.0% |
0.3% |
0.6% |
0.9% |
|
Anti-Jsa |
0.2% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Mia |
0.5% |
0.2% |
0.3% |
0.0% |
0.2% |
|
Anti-Joa |
0.0% |
0.2% |
0.0% |
0.0% |
0.5% |
|
Anti-Yta |
0.0% |
0.4% |
0.3% |
0.0% |
0.0% |
|
Anti-Mur |
0.0% |
0.2% |
0.0% |
0.0% |
0.0% |
|
Anti-PP1Pk |
0.0% |
0.2% |
0.0% |
0.0% |
0.2% |
|
Anti-Sc2 |
0.0% |
0.0% |
0.3% |
0.0% |
0.0% |
|
0.0% |
0.0% |
0.0% |
0.3% |
0.0% |
|
|
Anti-Dia |
0.0% |
0.0% |
0.0% |
0.0% |
0.7% |
|
Anti-Vel |
0.0% |
0.0% |
0.0% |
0.0% |
0.2% |
|
Anti-Hr |
0.0% |
0.0% |
0.0% |
0.0% |
0.2% |
Table 4: Perinatal Patient Antibody Titres Performed in Year 2021 and changed from Non-critical Level to Critical Level
|
Antibody |
Critical Level |
Non-Critical Level |
Non-Critical to Critical |
|---|---|---|---|
|
Anti-C |
1 |
14 |
0 |
|
Anti-c |
3 |
33 |
2 |
|
Anti-Ce |
1 |
5 |
0 |
|
Anti-CG |
1 |
4 |
1 |
|
Anti-Cw |
0 |
0 |
0 |
|
Anti-D |
16 |
23 |
4 |
|
Anti-D/C/G |
0 |
0 |
0 |
|
Anti-DC |
3 |
1 |
0 |
|
Anti-DE |
1 |
0 |
0 |
|
Anti-DG |
0 |
0 |
0 |
|
Anti-Dia |
0 |
0 |
0 |
|
Anti-E |
9 |
96 |
3 |
|
Anti-e |
0 |
4 |
0 |
|
Anti-Ec |
2 |
10 |
0 |
|
Anti-Fya |
3 |
11 |
0 |
|
Anti-Fyb |
0 |
0 |
0 |
|
Anti-G |
0 |
2 |
0 |
|
Anti-Jka |
1 |
36 |
0 |
|
Anti-Jkb |
0 |
4 |
0 |
|
Anti-Jk3 |
0 |
0 |
0 |
|
Anti-Joa |
0 |
1 |
0 |
|
Anti-K |
0 |
0 |
0 |
|
Anti-Kpa |
0 |
0 |
0 |
|
Anti-Lua |
0 |
1 |
0 |
|
Anti-Lub |
0 |
2 |
0 |
|
Anti-M |
2 |
35 |
1 |
|
Anti-Mia &/or Mur |
1 |
1 |
1 |
|
Anti-S |
1 |
10 |
0 |
|
Anti-s |
1 |
3 |
1 |
|
Anti-U |
0 |
0 |
0 |
|
Anti-Wra |
0 |
5 |
0 |
|
Anti-Vel |
1 |
0 |
0 |
|
Rh Antibody |
0 |
0 |
0 |
|
Anti-Cob |
0 |
0 |
0 |
|
Unidentified antibody |
0 |
0 |
0 |
|
Anti-PP1PK |
0 |
2 |
0 |
|
0 |
1 |
0 |
|
|
Anti-Dia |
0 |
1 |
0 |
|
Autoanti-E |
0 |
1 |
0 |
Table 5: Combination Antibodies Detected in Perinatal Patients in 2021
Reference Laboratory
The Reference Laboratory, Edmonton Diagnostic Services provides testing for hospital transfusion medicine laboratories. Hospital patients who are repeatedly transfused may develop red cell antibodies and as a result may have difficulty in tolerating transfusions. Diagnostic Services has specialized and experienced technologists that assist and provide consultation to hospital transfusion medicine laboratories. The Reference Laboratory identifies red cell antibodies, resolves blood group discrepancies, and performs direct antiglobulin testing, fetal bleed screening and other serological testing.
Staff within our department may collaborate with other references laboratories such as the National Immunohematology Reference laboratory (NIRL).
Diagnostic Services Red Cell Antibody Investigations
In 2021, hospitals referred 351 requests for red cell antibody identification.
Diagnostic Services provides support to hospitals in Alberta, Northwest Territories, northeastern British Columbia and Lloydminster, Saskatchewan. Referring hospitals have different capabilities and expertise in resolving red cell antibody investigations. Some hospitals have limited reagents for antibody identification or phenotyping of patient or donor units. Others have access to a wider variety of reagent red cell panels and methods as well as on site immunohematology expertise. A few hospital transfusion medicine laboratories have the resources to resolve the majority of serological problems and send only complex investigations for additional serological or genotyping studies.
Canadian Blood Services, Diagnostic Services provides consultation and testing support including antibody investigation, advanced or alternative techniques where required, and recommendations for compatibility testing methods and selection of appropriate donor unit phenotypes if necessary.
Reporting may include interim, final and supplemental reports, depending on the urgency of the testing, the need for patient transfusion and the complexity of the investigation. When a new antibody is identified by the Diagnostic Services Laboratory, a patient wallet card may be provided.
A. Testing Performed
The Reference Laboratory routinely performs the following tests:
- ABO/Rh blood type
- Screen for red blood cell antibodies
- Antibody Identification, if antibodies are detected
- Phenotyping
- Direct Antiglobulin Test
- Elution and Adsorption
- Cold Agglutinin Screen
Antibody Screening is routinely performed by solid phase testing. Combinations of solid phase testing and indirect antiglobulin tube testing using PEG for enhancement are the primary antibody identification methods. PEG IAT is also the manual back-up method for antibody screening. As a Reference Laboratory, the laboratory performs complex antibody investigations.
Figure 4: Total Reference Specimens Tested by Edmonton Reference Laboratory between 2017 and 2021
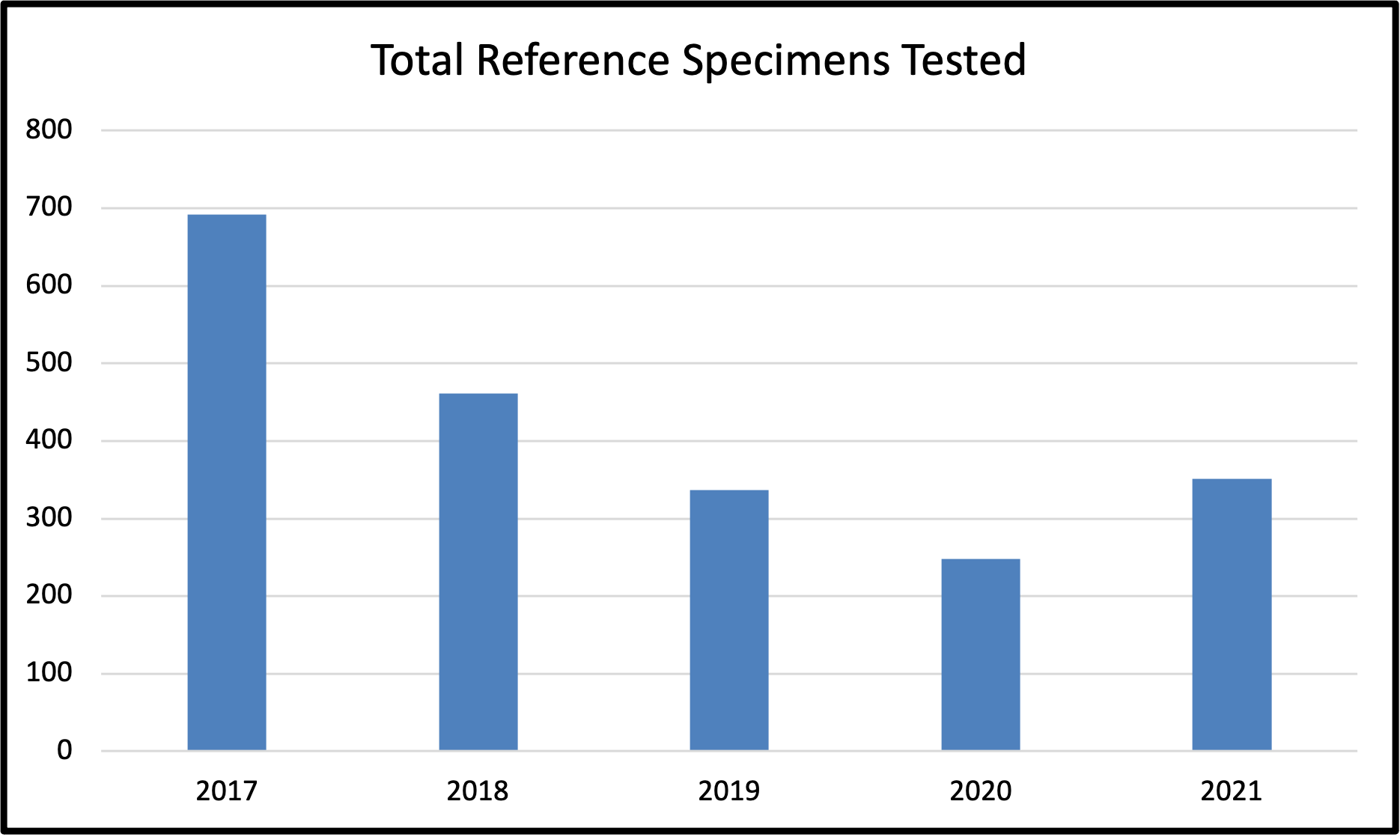
Table 6: Total Reference Specimens Tested by Edmonton Reference Laboratory between 2017 and 2021
|
Specimen Type |
2017 |
2018 |
2019 |
2020 |
2021 |
|
Total Reference Antibody Investigations |
692 |
461 |
337 |
248 |
351 |
B. Antibodies Identified
In 2021, a total of 169 antibodies were reported (see Table 7). The total number of antibodies detected is lower than in 2020, but the distribution of the most common antibodies remains consistent. One hundred and forty-one (141) patients had antibodies identified, and of these, forty-three (43) patients had multiple antibodies.
Antibodies identified are considered to be clinically significant if they have been reported to cause acute or delayed hemolytic transfusion reactions. The most common clinically significant antibodies identified were anti-D, anti-C, anti-E, anti-K and anti-Jka (see Figure 5) which together represented 23% of the total antibodies identified.
Figure 5: Total Number of Reference Antibodies Detected between 2017 and 2021
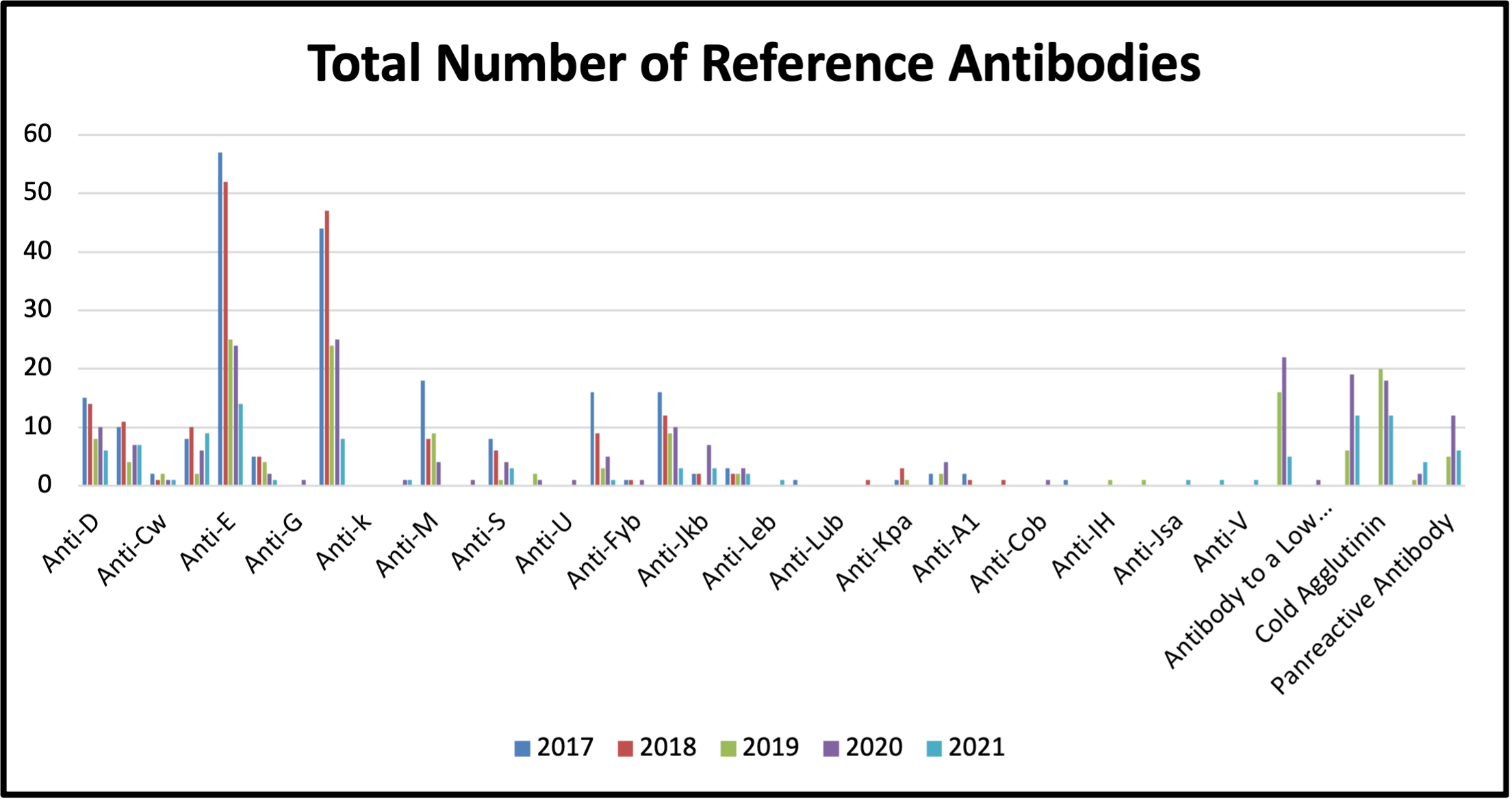
Table 7: Total Number of Reference Antibodies Detected between 2017 and 2021
|
Reference Antibodies Identified (Including Passive D) – *Prior to 2019 numbers included Crossmatch samples. |
|||||
|---|---|---|---|---|---|
|
Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|
Anti-D |
15 |
14 |
8 |
10 |
6 |
|
Anti-C |
10 |
11 |
4 |
7 |
7 |
|
Anti-Cw |
2 |
1 |
2 |
1 |
1 |
|
Anti-c |
8 |
10 |
2 |
6 |
9 |
|
Anti-E |
57 |
52 |
25 |
24 |
14 |
|
Anti-e |
5 |
5 |
4 |
2 |
1 |
|
Anti-G |
0 |
0 |
0 |
1 |
0 |
|
Anti-K |
44 |
47 |
24 |
25 |
8 |
|
Anti-k |
0 |
0 |
0 |
0 |
0 |
|
Anti-Kpa |
0 |
0 |
0 |
1 |
1 |
|
Anti-M |
18 |
8 |
9 |
4 |
0 |
|
Anti-N |
0 |
0 |
0 |
1 |
0 |
|
Anti-S |
8 |
6 |
1 |
4 |
3 |
|
Anti-s |
0 |
0 |
2 |
1 |
0 |
|
Anti-U |
0 |
0 |
0 |
1 |
0 |
|
Anti-Fya |
16 |
9 |
3 |
5 |
1 |
|
Anti-Fyb |
1 |
1 |
0 |
1 |
0 |
|
Anti-Jka |
16 |
12 |
9 |
10 |
3 |
|
Anti-Jkb |
2 |
2 |
0 |
7 |
3 |
|
Anti-Lea |
3 |
2 |
2 |
3 |
2 |
|
Anti-Leb |
0 |
0 |
0 |
0 |
1 |
|
Anti-Lua |
1 |
0 |
0 |
0 |
0 |
|
Anti-Lub |
0 |
0 |
0 |
0 |
0 |
|
Anti-Fy3 |
0 |
1 |
0 |
0 |
0 |
|
Anti-Kpa |
1 |
3 |
1 |
0 |
0 |
|
Anti-Wra |
2 |
0 |
2 |
4 |
0 |
|
Anti-A1 |
2 |
1 |
0 |
0 |
0 |
|
Anti-P1 |
0 |
1 |
0 |
0 |
0 |
|
Anti-Cob |
0 |
0 |
0 |
1 |
0 |
|
Anti Yta |
1 |
0 |
0 |
0 |
0 |
|
Anti-IH |
0 |
0 |
1 |
0 |
0 |
|
Anti-JMH |
0 |
0 |
1 |
0 |
0 |
|
Anti-Jsa |
0 |
0 |
0 |
0 |
1 |
|
Anti-Kpa |
0 |
0 |
0 |
0 |
1 |
|
Anti-V |
0 |
0 |
0 |
0 |
1 |
|
Panreactive Autoantibody |
0 |
0 |
16 |
22 |
5 |
|
Antibody to a Low Prevalence Antigen |
0 |
0 |
0 |
1 |
0 |
|
Unidentified Antibody |
0 |
0 |
6 |
19 |
12 |
|
Cold Agglutinin |
0 |
0 |
20 |
18 |
12 |
|
Autoantibody |
0 |
0 |
1 |
2 |
4 |
|
Panreactive Antibody |
0 |
0 |
5 |
12 |
6 |
|
0 |
0 |
0 |
88 |
67 |
|
|
Total |
212 |
186 |
148 |
281 |
169 |
*Not counted in previous years
Figure 6: Frequency of Reference Antibodies Detected between 2017 and 2021
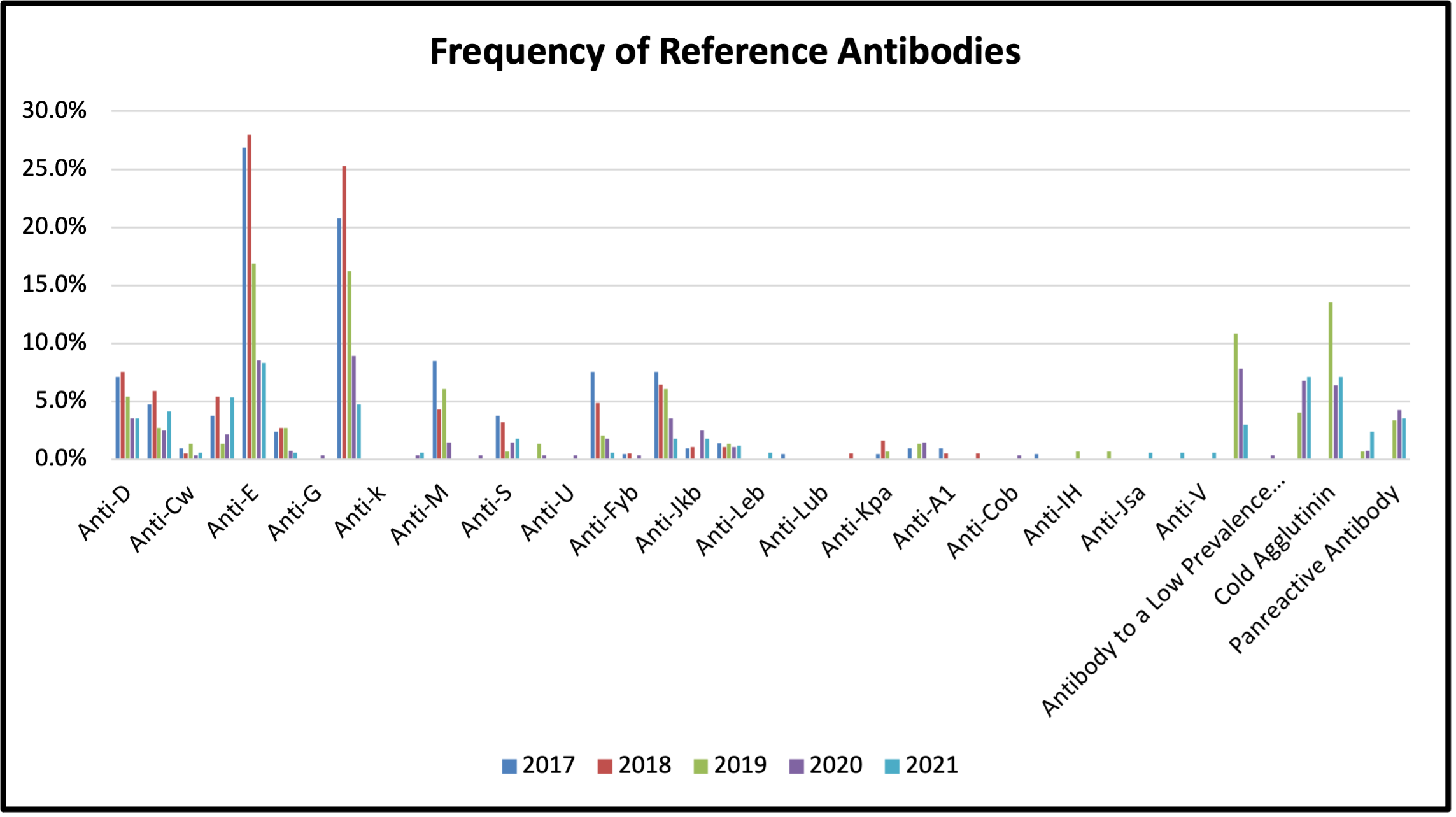
Table 8: Frequency of Reference Antibodies Detected between 2017 and 2021
|
Antibodies |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
Anti-D |
7.1% |
7.5% |
5.4% |
3.6% |
3.6% |
|
Anti-C |
4.7% |
5.9% |
2.7% |
2.5% |
4.1% |
|
Anti-Cw |
0.9% |
0.5% |
1.4% |
0.4% |
0.6% |
|
Anti-c |
3.8% |
5.4% |
1.4% |
2.1% |
5.3% |
|
Anti-E |
26.9% |
28.0% |
16.9% |
8.5% |
8.3% |
|
Anti-e |
2.4% |
2.7% |
2.7% |
0.7% |
0.6% |
|
Anti-G |
0.0% |
0.0% |
0.0% |
0.4% |
0.0% |
|
Anti-K |
20.8% |
25.3% |
16.2% |
8.9% |
4.7% |
|
Anti-k |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Kpa |
0.0% |
0.0% |
0.0% |
0.4% |
0.6% |
|
Anti-M |
8.5% |
4.3% |
6.1% |
1.4% |
0.0% |
|
Anti-N |
0.0% |
0.0% |
0.0% |
0.4% |
0.0% |
|
Anti-S |
3.8% |
3.2% |
0.7% |
1.4% |
1.8% |
|
Anti-s |
0.0% |
0.0% |
1.4% |
0.4% |
0.0% |
|
Anti-U |
0.0% |
0.0% |
0.0% |
0.4% |
0.0% |
|
Anti-Fya |
7.5% |
4.8% |
2.0% |
1.8% |
0.6% |
|
Anti-Fyb |
0.5% |
0.5% |
0.0% |
0.4% |
0.0% |
|
Anti-Jka |
7.5% |
6.5% |
6.1% |
3.6% |
1.8% |
|
Anti-Jkb |
0.9% |
1.1% |
0.0% |
2.5% |
1.8% |
|
Anti-Lea |
1.4% |
1.1% |
1.4% |
1.1% |
1.2% |
|
Anti-Leb |
0.0% |
0.0% |
0.0% |
0.0% |
0.6% |
|
Anti-Lua |
0.5% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Lub |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-Fy3 |
0.0% |
0.5% |
0.0% |
0.0% |
0.0% |
|
Anti-Kpa |
0.5% |
1.6% |
0.7% |
0.0% |
0.0% |
|
Anti-Wra |
0.9% |
0.0% |
1.4% |
1.4% |
0.0% |
|
Anti-A1 |
0.9% |
0.5% |
0.0% |
0.0% |
0.0% |
|
Anti-P1 |
0.0% |
0.5% |
0.0% |
0.0% |
0.0% |
|
Anti-Cob |
0.0% |
0.0% |
0.0% |
0.4% |
0.0% |
|
Anti Yta |
0.5% |
0.0% |
0.0% |
0.0% |
0.0% |
|
Anti-IH |
0.0% |
0.0% |
0.7% |
0.0% |
0.0% |
|
Anti-JMH |
0.0% |
0.0% |
0.7% |
0.0% |
0.0% |
|
Anti-Jsa |
0.0% |
0.0% |
0.0% |
0.0% |
0.6% |
|
Anti-Kpa |
0.0% |
0.0% |
0.0% |
0.0% |
0.6% |
|
Anti-V |
0.0% |
0.0% |
0.0% |
0.0% |
0.6% |
|
Panreactive Autoantibody |
0.0% |
0.0% |
10.8% |
7.8% |
3.0% |
|
Antibody to a Low Prevalence Antigen |
0.0% |
0.0% |
0.0% |
0.4% |
0.0% |
|
Unidentified Antibody |
0.0% |
0.0% |
4.1% |
6.8% |
7.1% |
|
Cold Agglutinin |
0.0% |
0.0% |
13.5% |
6.4% |
7.1% |
|
0.0% |
0.0% |
0.7% |
0.7% |
2.4% |
|
|
Panreactive Antibody |
0.0% |
0.0% |
3.4% |
4.3% |
3.6% |
|
Passive Anti-D |
0.0% |
0.0% |
0.0% |
31.3% |
39.6% |
Table 9: Combination Reference Antibodies Detected in 2021
|
Combination Antibodies |
Serology |
|---|---|
|
Anti-C Anti-e |
1 |
|
Anti-c Anti-Jka |
1 |
|
Anti-C Anti-K Unidentified Antibody |
1 |
|
Anti-C Cold Agglutinin |
1 |
|
Anti-Cw Unidentified Antibody Autoantibody |
1 |
|
Anti-D Anti-C |
3 |
|
Anti-D Anti-C Anti-V |
1 |
|
Anti-D Anti-E Anti-Jkb |
1 |
|
Anti-D Anti-Jkb |
1 |
|
Anti-E Anti-c |
2 |
|
Anti-E Anti-c Anti-K |
2 |
|
Anti-E Anti-c Anti-K Panreactive Autoantibody Autoantibody |
1 |
|
Anti-E Anti-c Anti-S |
1 |
|
Anti-E Anti-c Anti-S Anti-Jkb |
1 |
|
Anti-E Anti-c Cold Agglutinin |
1 |
|
Anti-E Anti-Jka |
1 |
|
Anti-E Anti-K |
1 |
|
Anti-E Anti-Kpa |
1 |
|
Anti-E Anti-S Anti-K Anti-Fya |
1 |
|
Anti-E Unidentified Antibody |
1 |
|
Anti-Jka Anti-Lea |
1 |
|
Anti-Jsa Cold Agglutinin |
1 |
|
Anti-K Anti-Leb |
1 |
|
Anti-K Unidentified Antibody |
1 |
|
Anti-Kpb Panreactive Antibody |
1 |
|
Anti-Lea Unidentified Antibody |
1 |
|
Cold Agglutinin Panreactive Antibody |
2 |
|
Cold Agglutinin Panreactive Autoantibody |
1 |
|
Cold Agglutinin Unidentified Antibody |
4 |
|
Cold Agglutinin Unidentified Antibody Panreactive Antibody |
2 |
|
1 |
|
|
Unidentified Antibody Panreactive Autoantibody |
1 |
|
Warm Autoantibody Panreactive Autoantibody |
2 |
Fetal Genotyping
Canadian Blood Services in Alberta refers specimens for fetal genotyping on prenatal plasma to the International Blood Group Reference Laboratory (IBGRL) of the National Health Services (NHS) in Bristol, United Kingdom. Amniotic fluid samples are rarely sent to the Versiti (formerly Blood Center of Wisconsin) for fetal genotyping. Testing on maternal blood samples is preferred because sample collection does not represent a risk to the fetus.
Specimens are submitted through the Maternal Fetal Medicine clinics in Edmonton or Calgary and are accepted if they meet the following criteria:
- The patient has an antibody capable of causing hemolytic disease of the fetus/newborn (HDFN), AND
- The partner is heterozygous for the corresponding antigen (or unknown), AND
- The antibody titre has reached a critical level, OR
- There has been a previous fetus/newborn affected by HDFN, OR
- The antibody is anti-K, OR
- Amniocentesis is being performed for another indication (ie advanced maternal age), even if the patient’s antibody titre is at a non-critical level.
Discussion with a Canadian Blood Services physician is required prior to submitting specimens for testing outside of these criteria.
The number of specimens referred for fetal genotyping has ranged between 18 and 24 specimens in recent years.
Table 10: Fetal Genotyping Results Summary between 2017 and 2021
|
2017 |
2018 |
2019 |
2020 |
2021 |
|
|---|---|---|---|---|---|
|
Total samples sent |
24 |
26 |
31 |
32 |
34 |
|
# of patients tested |
24 |
21 |
28 |
28 |
28 |
|
# of patients not requiring MFM follow-up. (Tested negative for the corresponding antigen) |
5 |
12 |
8 |
11 |
13 |
Table 11: Fetal Genotyping Results Summary from year 2021
|
Patient |
Perinatal Antibody |
Predicted Fetal Phenotype |
Follow-up Required |
|---|---|---|---|
|
1 |
Anti-E |
RhE Pos |
Yes |
|
2 |
Anti-D |
RhD Pos |
Yes |
|
3 |
Anti-E |
RhE Neg |
No |
|
4 |
Anti-D,C |
RhD Pos |
Yes |
|
5 |
Anti-K |
K Neg |
No |
|
6 |
Anti-K |
K Neg |
No |
|
7 |
Anti-D |
RhD Pos |
Yes |
|
8 |
Anti-D |
RhD Pos |
Yes |
|
9 |
Anti-D |
RhD Pos |
Yes |
|
10 |
Anti-E |
RhE Neg |
No |
|
11 |
Anti-D,C |
RhD Neg, RhC Neg |
No |
|
12 |
Anti-K |
K Neg |
No |
|
13 |
Anti-C |
C Neg |
No |
|
14 |
Anti-K |
K Neg |
No |
|
15 |
Anti-E |
RhE Pos |
Yes |
|
16 |
Anti-C |
RhC Pos |
Yes |
|
17 |
Anti-D,C |
RhD Pos |
Yes |
|
18 |
Anti-D |
RhD Pos |
Yes |
|
19 |
Anti-D |
RhD Pos |
Yes |
|
20 |
Anti-E |
RhE Pos |
Yes |
|
21 |
Anti-D,C |
Rh D Pos |
Yes |
|
22 |
Anti-K |
K Neg |
No |
|
23 |
Anti-K |
K Neg |
No |
|
24 |
Anti-D |
RhD Neg |
No |
|
25 |
Anti-E |
RhE Neg |
No |
|
Anti-D |
RhD Pos |
Yes |
|
|
27 |
Anti-D |
RhD Neg |
No |
|
28 |
Anti-E |
RhE Pos |
Yes |
RHD Red Cell Genotyping
Canadian Blood Services in Alberta provides RHD red cell genotyping for facilities in cases where the predicted RhD status of a patient cannot be determined due to discrepant, weak or inconclusive serological RhD testing. The following 2021 testing algorithm was used within Canadian Blood Services laboratories to determine which samples require RHD genotyping.
Text Version – Figure 7
Figure 7 describes RhD testing algorithm. If NEO/Manual Rh results and previous RHD Genotyping results are available, report RhD as per previous RHD Genotyping results.
If NEO/Manual Rh results are available but previous RHD Genotyping results are not available and Anti-D4 & Anti-D5 results are negative, check for the previous Novaclone Anti-D result in computer. If previous Novaclone Anti-D is Negative, report RhD as Rh Negative. If previous Novaclone Anti-D is positive, report as Rh Indeterminate and forward the sample for RHD Genotyping.
If NEO/Manual Rh results are available but previous RHD Genotyping results are not available and Anti-D4 & Anti-D5 results are negative, check for the previous Novaclone Anti-D result in computer. If no Novaclone Anti-D result is available in computer, perform Novaclone Anti-D Test. If Novaclone Anti-D is Negative, report RhD as Rh Negative. If Novaclone Anti-D tests positive, repeat Novaclone Anti-D Test with Novaclone Control. If repeat Novaclone Anti-D is positive, report as Rh Indeterminate and forward the sample for RHD Genotyping.
If NEO/Manual Rh results are available but previous RHD Genotyping results are not available and Anti-D4 & Anti-D5 results are less than or equal to one or equivocal or greater than or equal to two grade difference, perform tube tests for Anti-D4, Anti-D5, Monoclonal Control, Novaclone Anti-D and Novaclone Control. If any Rh Antisera results are less than or equal to one grade difference or greater than or equal to two grade difference, report as Rh Indeterminate and forward the sample for RHD Genotyping. If all the Rh antisera results are greater than or equal to two with less than or equal to one grade difference, report RhD as Rh Positive. If all antisera results are negative, report RhD as Rh Negative.
If NEO/Manual Rh results are available but previous RHD Genotyping results are not available and Anti-D4 & Anti-D5 results are greater than or equal to two, report RhD as Rh Positive.
Table 12: Patient # - RHD Type/Result in year 2021
|
2021 RHD Genotyping Results |
|
|---|---|
|
RHD Variant |
Number Identified |
|
Normal RHD |
619 |
|
Weak D type 1 |
232 |
|
Weak D type2 |
120 |
|
Weak D type 3 |
59 |
|
Weak D type 4.0 or 4.3 |
53 |
|
Weak D type 4.1 |
7 |
|
RHD Deletion |
24 |
|
DAR |
56 |
|
RHD psi (Pseudogene) |
12 |
|
DAU2 |
11 |
|
DAU3 |
8 |
|
DAU4 or DV type 5 |
5 |
|
DAU5 or DV type1 or DBS2 |
2 |
|
DCSI or DFV |
3 |
|
DFR or DFR3 |
3 |
|
DHMi |
3 |
|
DIIIa or DIIIa-CE(4-7)-D |
10 |
|
DIIIc |
1 |
|
DOL or DOL2 |
6 |
|
DV type 2 or DBS1 |
2 |
|
DVI |
6 |
|
DNB |
2 |
|
DHMi |
3 |
|
1227A (Del) |
1 |
|
Weak D type 5 |
3 |
|
RHD-CE(3-9)-D |
1 |
|
Normal RHD with a variant allele (D+) |
112 |
|
Heterozygous alleles (D+) |
4 |
|
Heterozygous variant alleles (D-) |
14 |
|
Sent for Sequencing |
77 |
|
Total |
1459 |
The array used for RHD genotyping (Immucor’s BioArray BeadChip™ Molecular Assay) is extensive and can detect the most common mutations of the RHD gene. The most common variants detected are weak D type 1, weak D type 2 and weak D type 3. Individuals with these phenotypes can be safely treated as Rh positive as it has been established that they will not form alloanti-D. Patients with any other weak or partial D phenotypes may be capable of forming alloanti-D and should be treated as Rh negative. When none of the variants present on the array are detected, the software will produce a result of “Possible D”. Prior to 2018, Canadian Blood Services recommended that patients with a result of “Possible D” be treated as Rh negative. However, based on clinical experience at that time, the reporting was changed in 2018 such that patients with a result of “Possible D” were reported as Rh Positive. Categorization of “Possible D” individuals is under continuing review as more experience is gained with the assay, and sequencing to resolve difficult cases becomes more readily available.
Table 13: RHD Genotyping – Number of Rh Positive and Rh Negative Predicted Phenotypes Between 2017 and 2021
|
Rh Positive/Rh Negative |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
309 |
581 |
702 |
937 |
1034 |
|
|
Rh Negative |
390 |
153 |
280 |
209 |
425 |
|
Total # samples tested |
699 |
734 |
982 |
1146 |
1459 |
A. Turnaround Times
To ensure timely reporting of patient test results, Canadian Blood Services monitors turnaround time (TAT) from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available. Since monitoring of this quality indicator began in 2008, the percentage of perinatal specimens has been close to the predefined TAT threshold. The percentage of reference specimens has consistently met the predefined TAT threshold. Samples whose testing failed to meet expected TATs are usually those where clinically significant antibodies are detected or where difficulty in finding compatible blood is encountered.
Table 14: Turnaround Time from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available – Routine Criteria by Specimen Type
|
Specimen Type |
Expected Turnaround Time |
Expected % of Specimens Which Meet or Exceed Expected TAT |
|---|---|---|
|
72 hours |
85% |
|
|
Reference Testing |
72 hours |
85% |
Table 15: Turnaround Time from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available – Perinatal Routine TAT between 2017 and 2021
|
Turnaround Time (TAT) |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
80% |
88% |
85% |
85% |
83% |
|
|
% of Specimens Tested > 72 hours |
20% |
12% |
15% |
15% |
17% |
Table 16: Turnaround Time from when specimens are received at Canadian Blood Services in Edmonton to the time when the results are available – Reference Specimens TAT between 2017 and 2021
|
Turnaround Time (TAT) |
2017 |
2018 |
2019 |
2020 |
2021 |
|---|---|---|---|---|---|
|
99% |
99% |
100% |
100% |
100% |
|
|
% of Specimens Tested > 72 hours |
1% |
1% |
0% |
0% |
0% |
Figure 8: Turnaround Time for Perinatal Routine Samples between 2017 and 2021
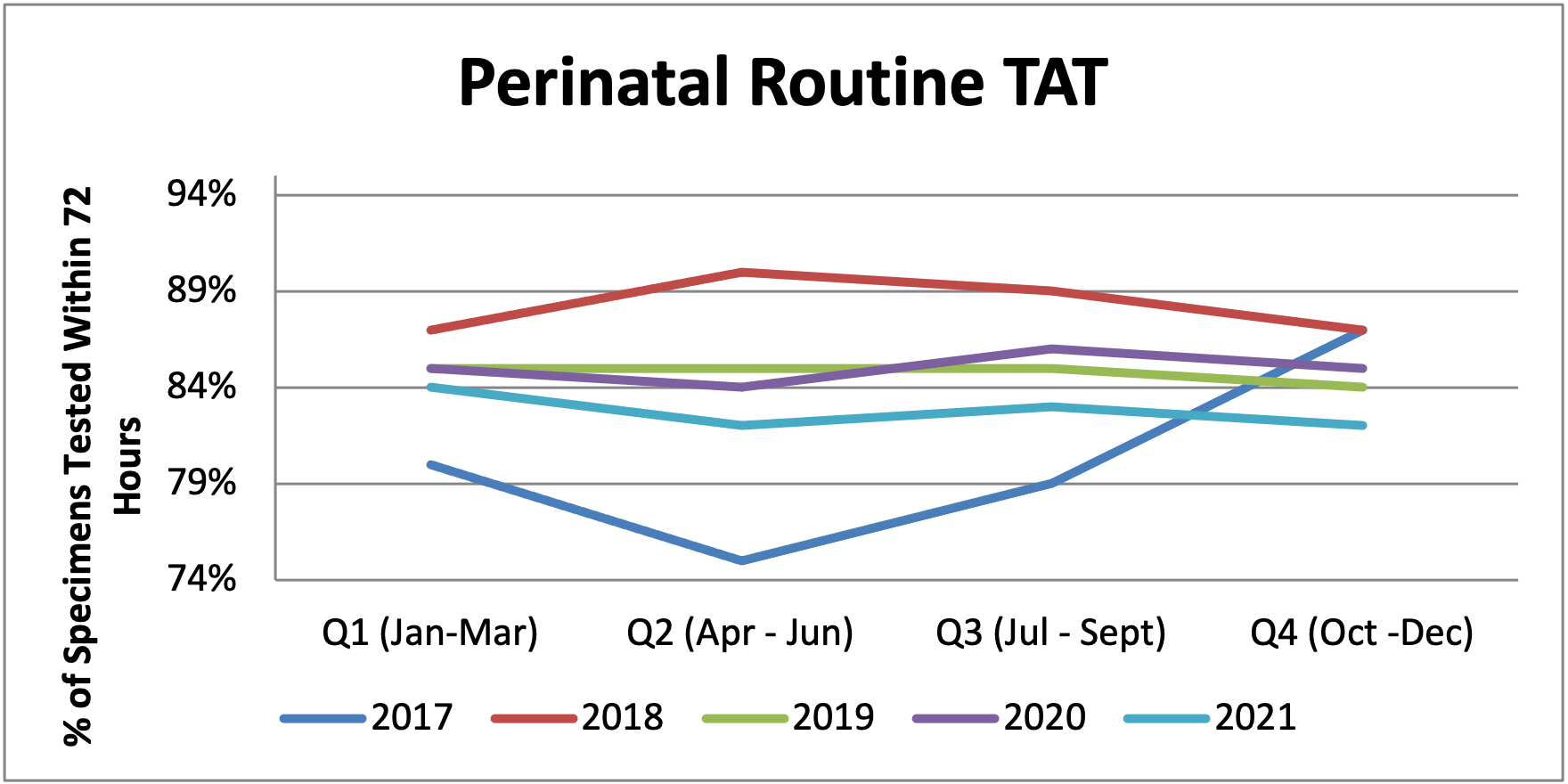
Table 17: Turnaround Time for Perinatal Routine Samples between 2017 and 2021
|
Year |
Q1 (Jan-Mar) |
Q2 (Apr - Jun) |
Q3 (Jul - Sept) |
Q4 (Oct -Dec) |
%<72 hours |
%>72 hours |
|---|---|---|---|---|---|---|
|
2017 |
80% |
75% |
79% |
87% |
80% |
20% |
|
2018 |
87% |
90% |
89% |
87% |
88% |
12% |
|
2019 |
85% |
85% |
85% |
84% |
85% |
15% |
|
85% |
84% |
86% |
85% |
85% |
15% |
|
|
2021 |
84% |
82% |
83% |
82% |
83% |
17% |
Figure 9: Turnaround Time for Reference Testing between 2017 and 2021
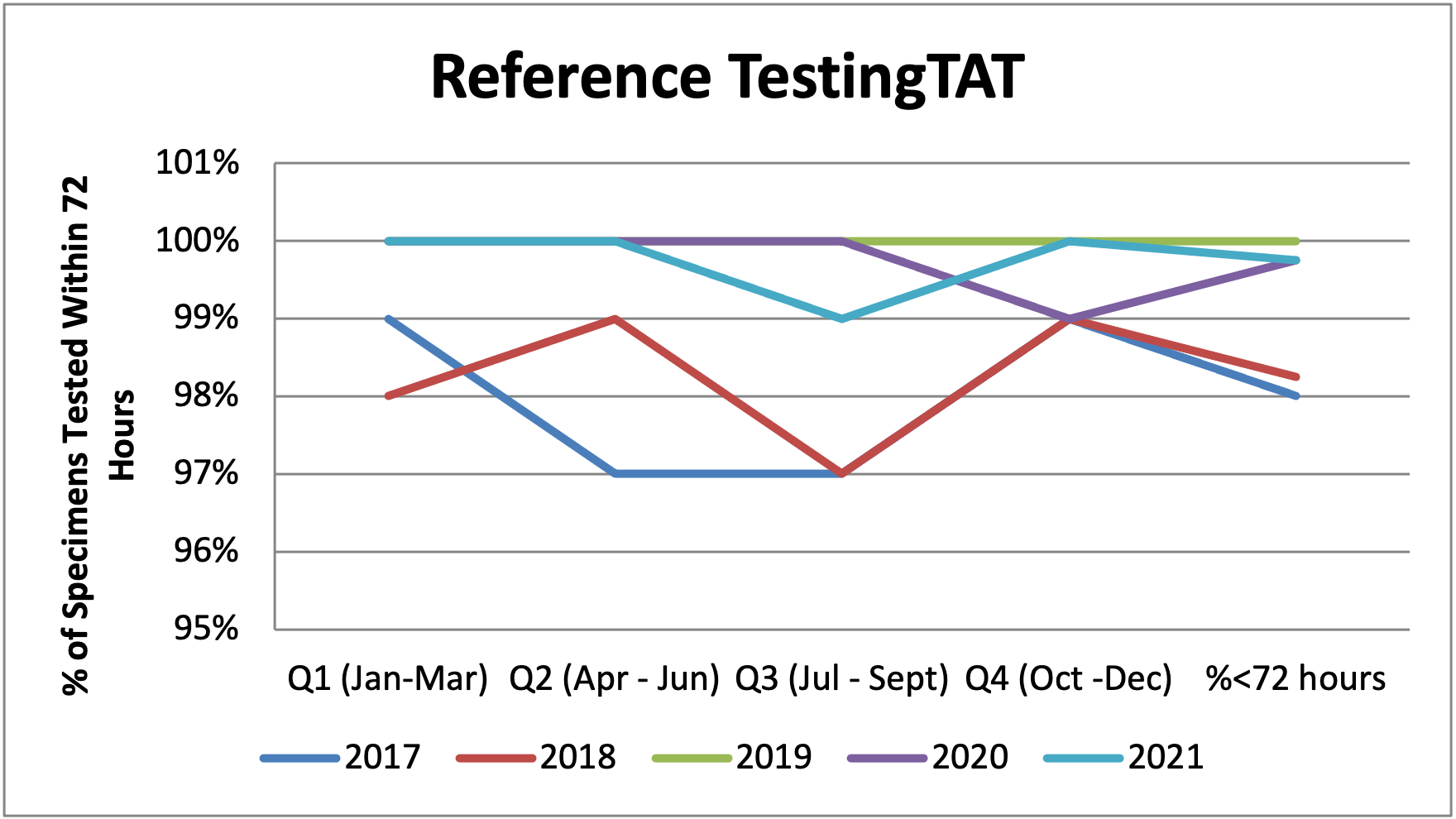
Table 18: Turnaround Time for Reference Testing between 2017 and 2021
|
Year |
Q1 (Jan-Mar) |
Q2 (Apr - Jun) |
Q3 (Jul - Sept) |
Q4 (Oct -Dec) |
%<72 hours |
%>72 hours |
|---|---|---|---|---|---|---|
|
2017 |
99% |
97% |
97% |
99% |
98% |
2% |
|
2018 |
98% |
99% |
97% |
99% |
98% |
2% |
|
2019 |
100% |
100% |
100% |
100% |
100% |
0% |
|
100% |
100% |
100% |
99% |
100% |
0% |
|
|
2021 |
100% |
100% |
99% |
100% |
100% |
0% |
B. Rejected Specimens
Each time a specimen is rejected, a reason for rejection is entered into our Laboratory Information System. This data is then retrieved and analysed on a quarterly basis for both reference samples which are coming from hospitals and for perinatal samples which are primarily collected at community collection sites. The Diagnostic Services Laboratory is following the provincial specimen rejection guidelines for Alberta.
The reasons for rejecting specimens in the reference and the perinatal laboratories are somewhat different.
For perinatal specimens, the most common reasons for rejecting a sample for testing are patient identification labelling errors and duplicate requests for testing (duplicate specimens). Testing requests are rejected if another sample from the patient was tested within the previous 96 hours. Often a duplicate test request sample is collected when the patient has seen their family physician and then sees their obstetrician shortly after. We do ensure that the report from the initial testing is sent to the second physician making the request. Health care professionals can access Canadian Blood Services reports for Alberta patients on Netcare, Alberta’s Electronic Health Record.
Figure 10: Perinatal Rejection Reasons in Year 2021
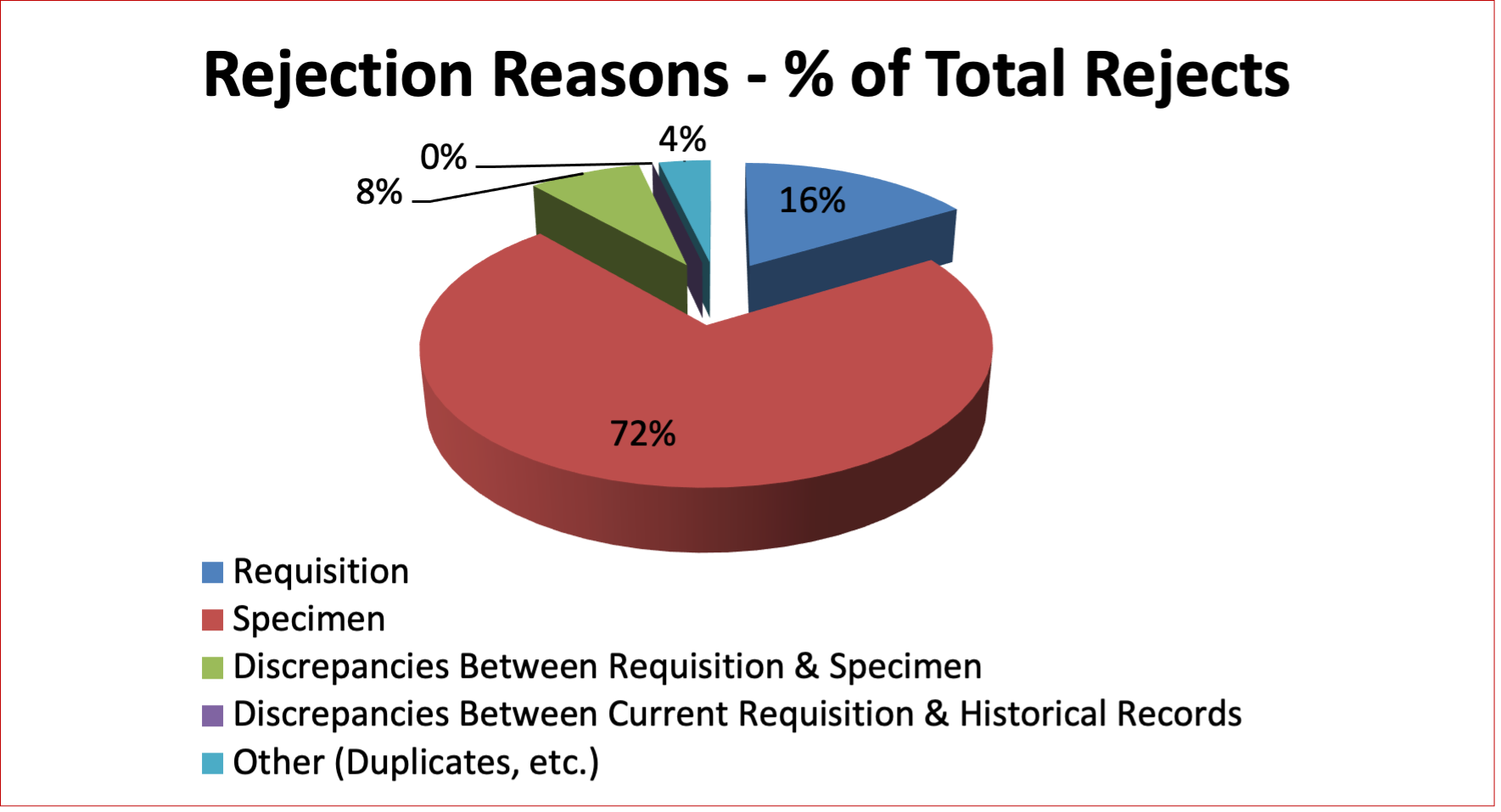
Table 19: Quarterly Rejection Rates – Perinatal Specimens in Year 2021
|
Rejection Category |
Annual Totals |
% of Total Rejects |
Q1 |
Q2 |
Q3 |
Q4 |
|---|---|---|---|---|---|---|
|
Requisition |
81 |
16% |
21 |
33 |
19 |
8 |
|
Specimen |
357 |
72% |
124 |
85 |
105 |
43 |
|
Discrepancies Between Requisition & Specimen |
39 |
8% |
7 |
14 |
14 |
4 |
|
Discrepancies Between Current Requisition & Historical Records |
0 |
0 |
0 |
0 |
0 |
0 |
|
Other (Duplicates, etc.) |
18 |
4% |
4 |
5 |
6 |
3 |
|
495 |
- |
156 |
137 |
144 |
58 |
|
|
Total # specimens received |
54962 |
- |
18372 |
15007 |
16228 |
5355 |
|
Rejections as a % of total |
0.9% |
- |
0.8% |
0.9% |
0.9% |
1.1% |
Figure 11: Reference Rejection Reasons in Year 2021
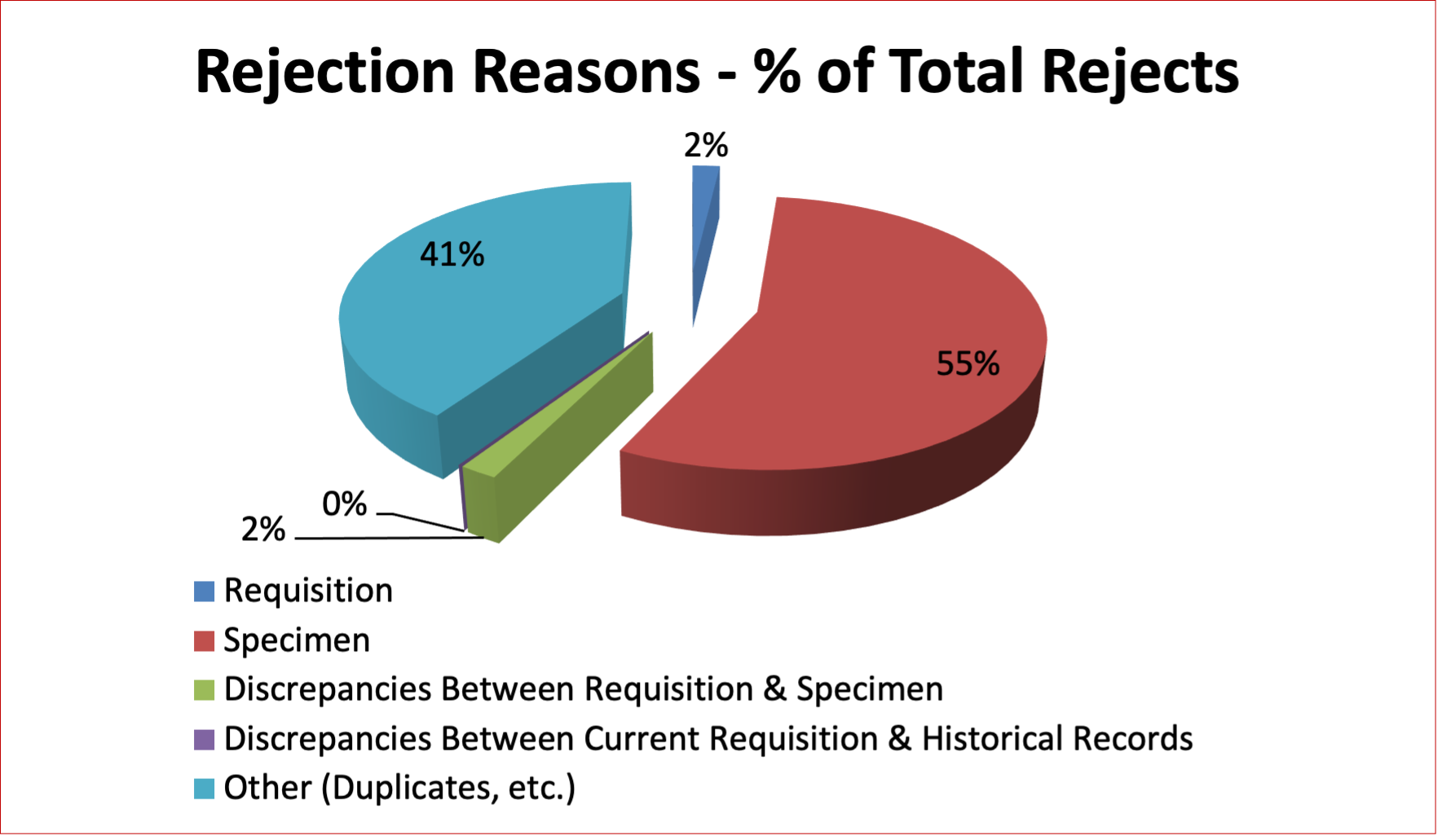
Table 20: Quarterly Rejection Rates – Reference in Year 2021
|
Rejection Category |
Annual Totals |
% of total Rejects |
Q1 |
Q2 |
Q3 |
Q4 |
|---|---|---|---|---|---|---|
|
Requisition |
2 |
2% |
1 |
1 |
0 |
0 |
|
Specimen |
60 |
56% |
8 |
16 |
14 |
22 |
|
Discrepancies Between Requisition & Specimen |
2 |
2% |
1 |
1 |
0 |
0 |
|
Discrepancies Between Current Requisition & Historical Records |
0 |
0 |
0 |
0 |
0 |
0 |
|
Other (Duplicates, etc.) |
44 |
41% |
14 |
10 |
9 |
11 |
|
108 |
- |
24 |
28 |
23 |
33 |
|
|
Total # specimens received |
2319 |
- |
623 |
650 |
524 |
522 |
|
Rejections as a % of total |
4.7% |
- |
3.9% |
4.3% |
4.4% |
6.3% |
Diagnostic Services Updates 2021
Updates pertain to all Diagnostic Services sites within Canadian Blood Services: Vancouver, Edmonton, Winnipeg, and Brampton
|
ALL |
NEO Iris Analyzer implemented April to June 2021. Eight NEO Instruments in Diagnostic Services were replaced with the next generation NEO IRIS instrument. NEO Iris performs ABO/RH and antibody testing.
|
|
|
Edmonton |
Edmonton DS obtained the CPSA 4-year accreditation on 20021-02-25. |
|
|
Edmonton |
Transfer of HEA and RHCE genotype testing to Brampton, 2021-10-01 |
|
|
Vancouver |
Awarded CAP Accreditation Dec 2021. |
|
|
Vancouver |
CPSBC – DAP ISO 15189 Audit. ISO 15189 accreditation pending final acceptance. |
|
|
Winnipeg |
Preparation for implementation of the Canadian Blood Services satellite Lab at St Boniface Hospital in March 2022. The Lab will act as a contingency site for services delivered by Winnipeg Diagnostic Services. |
|
|
Winnipeg |
Implementation of equipment in NPIRL – Multisizer 3 (cell counter) and thermocyclers |
|
|
Management of supplies, inventory and testing to ensure provision of services are not impacted during supply chain issues experienced in a pandemic. |
||
|
Winnipeg |
eTraceLine environments (perinatal and Crossmatch) were merged to allow better efficiency and ease of use for the labs now that staff are cross trained. |
|
|
Winnipeg |
Project to implement HistoTrac LIS and replace the access database currently used in Winnipeg in 2021. Projected implementation is January 2023. |
|
Presentations / Abstracts / Publications Listing
|
Lhevinne Ciurcovich, Lynnette Beaudin, Arianne Fuellos, Balkar Gill, Ilona Resz, Debra Lane, Judith Hannon, Gwen Clarke, Melanie Bodnar. Comparison of Manual SIAT vs Automated Solid Phase Methodology for Perinatal Antibody Titration. Poster, CSTM 2021 |
|
Lhevinne Ciurcovich1, Sarah Manfredi2, Sarah Buchko2, Darlene Mueller2, Michelle Wong2, Mohammad Bahmanyar2, MatthewYan1, Gwen Clarke1. Anti-Ina Implicated in Hemolytic Disease of the Fetus and Newborn in an Indigenous Woman. Poster, CSTM 2021 1: Canadian Blood Services, BC and Yukon Centre 2: Fraser Health Authority, British Columbia |
|
Lhevinne Ciurcovich, Gwen Clarke, Matthew Yan. A Case of ABO Chimerism in a Perinatal Patient. Poster, CSTM 2021 |
|
Lhevinne Ciurcovich. Cell-Free Fetal DNA Testing: Advantages, Challenges and Limitations. Presentation: Virtual Conference, 22nd Annual Education Day on Blood Transfusion Issues, 2021-09-24. |
|
Lhevinne Ciurcovich. Immunohematology Case Studies. Presentation: Immucor ImmuTECH Education Day (Virtual) 2021-05-05. |
|
Lynnette Beaudin, Dr. Lani Lieberman MD, FRCP, Fetal and neonatal alloimmune thrombocytopenia (FNAIT): Diagnosis, Investigation and Treatment. Presentation: U of T Monthly Transfusion Rounds (Virtual) 2021-02-25 |
|
Bodnar M, Hannaford K, Montemayor-Garcia C, Hannon J. Blindspots in Immucor BioArray RHD Molecular BeadChip Test: A Review of Cases at Canadian Blood Services Referred Out for RHD Gene Sequencing. Poster/Abstract, CSTM 2021 |
|
Floch A, Vege S, Berardi P, Hannon J, Ochoa-Garay G, Lomas-Francis C, et al. A change in RHD is associated with aberrant transcription and very weak D phenotype. Transfusion 2021 |
|
Flegel WA, Bodnar M, Clarke G, Hannon J, Lieberman L., ‘What constitutes the most cautious approach for a pregnant person with weak D type 4.0?’ Letter to the Editor, CMAJ June 2021 |
